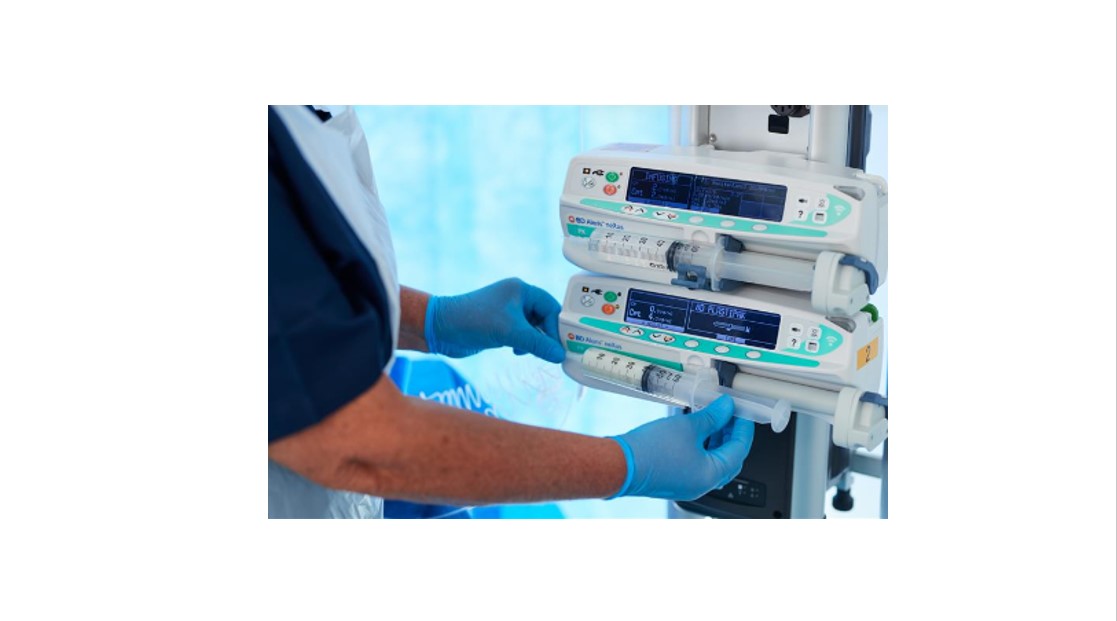
New clinical safety tips for syringe pump systems for microinfusion
Though advances in technology have brought improvements in education, there is still a general lack of awareness among healthcare workers around the proper use of syringe pump systems for microinfusion and their mechanics.1
This lack of awareness remains a considerable patient risk, posing various safety concerns.1
To support patient safety improvements through awareness and education, Weiss et al. worked with an expert group to put together a comprehensive guide of clinical tips for using syringe pump systems for microinfusion IV drug therapy.1
Microinfusion vs. macroinfusion—what’s the difference?
Macroinfusion relates to the intravenous administration of highly concentrated drugs at higher flow rates of 7-50mL/hour.1,2
Microinfusion, on the other hand, was designed to minimise fluid delivery associated with drug administration, with lower flow rates of 0.1-10mL/hour.1,2
This technique is typically used in critical care medicine and anaesthesia to prevent fluid overload in neonates and infants as well as patients receiving multiple infusions.1
Challenges with syringe pump systems for microinfusion
Flow rate variability has persisted as a significant challenge when using syringe pump systems for microinfusion.1
In particular, low flow rates when using microinfusion pumps for IV administration of highly concentrated, short-acting physiologically potent drugs come with serious clinical risks, including:1
• Delayed drug delivery: Can be caused by syringe infusion pump start-up, infusion line dead spaces
• Inaccurate drug delivery: Resulting from the use of nonvalidated syringes
• Irregular drug delivery: Such as free flow, during/after syringe infusion pump changeover, vertical displacement of the infusion pump
• Inadvertent infusion line occlusion: With undetected interruption of IV drug delivery and risk of release drug bolus administration
• Dosing errors: Due to flow rate variability in multi-infusion syringe pump setups
Learn more about syringe pump safety: Is your syringe intravenous (IV) infusion system safe for your patients?
Clinical recommendations for syringe infusion pump system usage
Throughout their recommendations, Weiss et al. place a focus on education and practical tips to implement the most effective syringe infusion pump system.1
From training all users on their specific system, to establishing and maintaining proper protocol for the management of IV drug infusions, Weiss et al. insist that continuing education is needed to prevent adverse events related to the shortcomings of IV drug infusion systems.1
The authors go on to suggest other ways of optimising syringe pump systems, such as using the smallest appropriate size luer Lok syringe, keeping the compliance and resistance of the infusion pump system as low as possible and minimising the number of infusion pumps connected to the same venous catheter lumen.1
They also highlight the importance of using a syringe that has been validated by the pump manufacturer.1
The use of nonvalidated syringes can result in as low as 10% under delivery and up to 24% over delivery of medication.3
Furthermore, the availability of more than one brand of syringe compounds the risk of adverse IV drug infusion events.3
To combat this, Weiss et al. recommend establishing a simple syringe library per microinfusion pump to help decrease selection errors.1
They also call on manufacturers to demonstrate infusion-pump-syringe compatibility.1
Learn more about validated pump syringes: Three reasons to choose a validated BD Plastipak™ syringe for use with infusion pumps
Start implementing safer IV drug infusion practices
When adhered to, the 10 tips outlined by Weiss et al can help you and your clinical teams support safer infusions.1
They conclude that, “vigilance, strict protocols and optimally designed and assembled equipment are needed for advancing patient safety using syringe infusion pump systems for IV drug therapy.”1
Register to download the "10 safety recommendations from pump to patient" infographic
References
1. Weiss M, van der Eijk A, Lonnqvist PA, Lucchini A, Timmermann A. 10 clinical tips for advancing patient safety when using syringe pump systems for microinfusion intravenous drug therapy. Eur J Anaesthesiol. 2023 Jun;40(6):387-390. DOI: 10.1097/EJA.0000000000001839.
2. Kim UR, Peterfreund RA, Lovich MA. Drug infusion systems: technologies, performance, and pitfalls. Anesthesia & Analgesia. 2017 May;124(5):1493-1505. DOI: 10.1213/ANE.0000000000001707.
3. Tooke LJ, Howell L. Syringe drivers: incorrect selection of syringe type from the syringe menu may result in significant errors in drug delivery. Anaesth Intensive Care. 2014 Jul;42(4):467-72. DOI: 10.1177/0310057X1404200407.
This list of references to third-party peer-reviewed material and the sites they are hosted on are provided for your reference and convenience only, and do not imply any review or endorsement of the material or any association with their operators. The Third-Party References (and the Web sites to which they link) may contain information that is inaccurate, incomplete, or outdated. Your access and use of the Third Party Sites (and any Web sites to which they link) is solely at your own risk.


BD-92134