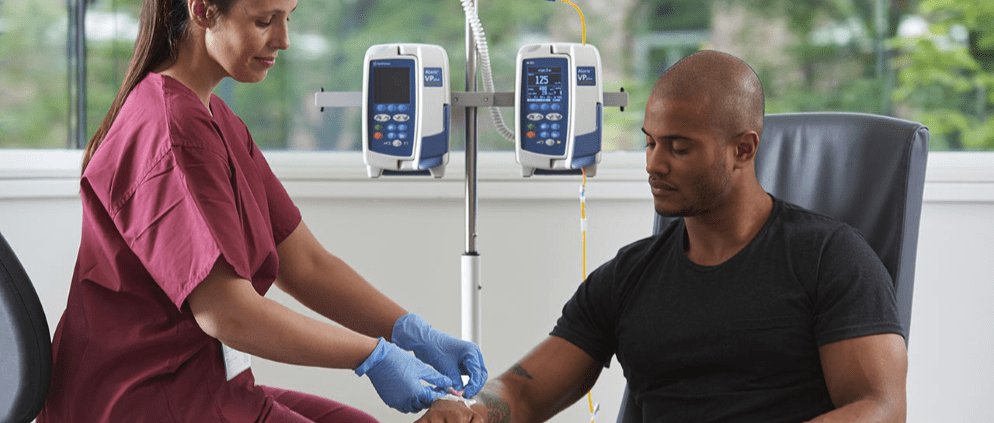
EU Directive 2022/431: What’s in it for healthcare workers?
EU Directive 2022/431 in a nutshell
European Union (EU) Directive 2022/431, also known as the Carcinogens, Mutagens and Reprotoxic Substances Directive (EU-CMRD) aims to protect healthcare workers (HCWs) from the risks associated with occupational exposure to carcinogens, mutagens and reprotoxic substances.1
Did you know?
- 8 million healthcare workers are exposed to hazardous drugs (HDs) in the EU2
- Close to 67% of EU workers exposed to carcinogens died from cancer in 20163
- Cancer costs Europe an estimated €100 billion annually4
How EU Directive 2022/431 differs from Directive 2004/37/EC
This directive amends Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens and mutagens (EU-CMD) at work by including HDs or hazardous medicinal products.1 HDs which contain one or several CMRs fall under the scope of this directive.1
Another important change to this directive is the inclusion of reprotoxic substances.5 Reprotoxic applies to substances that interfere with reproduction (e.g., infertility, miscarriages and foetal malformations) as well as causing toxicity in offspring.1,6
The directive’s original title was changed from the protection of workers from the risks related to exposure to carcinogens or mutagens (EU-CMD) at work to the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances (EU-CMRD) at work.5
More on this topic: New clinic study: Reduce hazardous drug contamination with BD
What does this directive mean for healthcare settings?
The directive plays a crucial role in ensuring the health and safety of workers in the EU, and its implementation is a key responsibility for all EU countries. In the healthcare sector, workers can be exposed to carcinogens, mutagens, and reprotoxic substances through various sources, such as certain medications, cleaning products or chemical agents.2,6
The inclusion of hazardous medicinal products in the directive means that healthcare facilities will need to assess the risk of exposure to these substances and implement appropriate protective measures.1
Key EU Directive 2022/431 healthcare recommendations1
Employers are responsible for:
- Performing regular risk of exposure assessments
- Providing mandatory high-quality training to HCWs who may be exposed to HDs
- Using appropriate procedures to measure hazardous substances
- Adopting a closed technological system when HDs cannot be replaced
- Involving workers and/or workers’ representatives in applying this directive
These measures fall under the categories “engineering controls” and “administrative controls” listed in the hierarchy of controls below. By implementing a hierarchy of controls, you can help protect your colleagues’ safety and yours in the workplace.7
Hierarchy of controls from most effective to least effective7
- Elimination: Remove the HD
- Substitution: Replace the HD
- Engineering controls: Isolate HCWs from HDs
- Administrative controls: Implement processes to help reduce the duration, frequency or intensity of exposure to HDs
- Personal protective equipment (PPE): Wear these to minimise exposure to HDs
More on this topic: New nursing guidelines recommend more frequent surface monitoring
The future of this directive
5th April 2024: EU member states should transpose the EU-CMRD into national law.1
5th April 2025: The European Commission should provide a list of HDs that will be classified as category 1A or 1B carcinogens, mutagens or reprotoxic substances as set out in Annex I to Regulation (EC) No 1272/2008.1
“The EU is taking another step to keep workers safe from substances which may lead to cancer or other illnesses. These new rules will reduce exposure to cancer-causing chemicals for an estimated one million workers,” according to Janez Cigler Kralj, the former Slovenian minister for labour, family, social affairs and equal opportunities.5
For highlights of EU Directive 2022/431, here’s a useful infographic from the Safe Handling of Hazardous Drugs hub. Watch the video below to find out more on HCW risk of exposure to HDs handled as a part of daily patient care and how following the measures outlined in the EU-CMRD helps ensure HCW safety while working.
References
- European Parliament. DIRECTIVE (EU) 2022/431 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 9 March 2022 Amending Directive 2004/37/EC on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work.; 2022. Accessed February 13, 2024. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022L0431#:~:text=This%20Directive%20has%20as%20its,the%20prevention%20of%20such%20risks.
- European Commission. Study Supporting the Assessment of Different Options Concerning the Protection of Workers from Exposure to Hazardous Medicinal Products, Including Cytotoxic Medicinal Products.; 2021. Accessed February 13, 2024. https://op.europa.eu/en/publication-detail/-/publication/f43015ec-a24f-11eb-b85c-01aa75ed71a1
- European Agency for Safety and Health at Work (EU-OSHA). Work-related cancer. Published 2024. Accessed February 13, 2024. https://osha.europa.eu/en/themes/work-related-diseases/work-related-cancer
- Directorate-General for Health and Food Safety. Europe’s Beating Cancer Plan: Communication from the Commission to the European Parliament and the Council. European Commission; 2021. Accessed February 13, 2024. https://health.ec.europa.eu/document/download/26fc415a-1f28-4f5b-9bfa-54ea8bc32a3a_en?filename=eu_cancer-plan_en_0.pdf
- Council of the European Union. EU to improve protection of workers from dangerous chemical substances. Published December 16, 2021. Accessed February 13, 2024. https://www.consilium.europa.eu/en/press/press-releases/2021/12/16/eu-to-improve-protection-of-workers-from-dangerous-chemical-substances/
- European Agency for Safety and Health at Work (EU-OSHA). Carcinogenic, mutagenic, reprotoxic (CMR) substances. Published June 2, 2022. Accessed February 13, 2024. https://oshwiki.osha.europa.eu/en/themes/carcinogenic-mutagenic-reprotoxic-cmr-substances
- National Institute for Occupational Safety and Health (NIOSH). Hierarchy of Controls. US Centres for Disease Control and Prevention (CDC). Published January 17, 2023. Accessed February 13, 2024. https://www.cdc.gov/niosh/topics/hierarchy/default.html
This list of references to third-party peer-reviewed material and the sites they are hosted on are provided for your reference and convenience only, and do not imply any review or endorsement of the material or any association with their operators. The Third-Party References (and the Web sites to which they link) may contain information that is inaccurate, incomplete, or outdated. Your access and use of the Third Party Sites (and any Web sites to which they link) is solely at your own risk.
BD-115956