This consensus project was initiated in light of the dramatic changes in PVADs that have occurred over the last decade, including1:
- Updated recommendations from Evidence-based Prevention and Infection Control (EPIC) and the Standards of Practice of the Infusion Nursing Society (INS) for the insertion and management of PVADs
- Awareness of different indications between peripheral and central VADs
- The introduction of integrated SPCs associated with less risk and long peripheral catheters (LPCs)
- The development of new technologies for the insertion of peripheral VADs
- New algorithms for a proper choice of VADs
Methods
A panel of 11 experts in venous access from different European countries was selected by the President and the Chairman of the Scientific Committee of WoCoVA. A bibliography of studies performed between Jan 2013 to Dec 2018, focusing on PVADs and including both retrospective and prospective clinical studies, was forwarded to all the panelists. Five working groups with 2–3 experts were defined for each of these PVAD-related topics:
- Classification and definition
- Indications
- Insertion
- Maintenance
- Removal
Each working group had the task of reviewing the literature on its assigned topic and producing answers to specific questions that were previously developed by the whole panel. These questions were:
Group 1:
- What is the most appropriate definition of a peripheral VAD?
- What is the most appropriate classification to describe the different types of peripheral VADs?
Group 2:
- What are the different indications for peripheral versus central VAD, taking into account clinical performance and the risk of complications?
- What are the most appropriate indications for the different types of peripheral VADs in the adult patient, considering clinical performance and the risk of complications?
Group 3:
- What is the role of site selection in reducing insertion-related complications?
- What is the most appropriate insertion strategy for reducing the risk of infection?
- What is the most appropriate strategy for securing the peripheral VAD?
- What is the role of ultrasound guidance when inserting a peripheral VAD?
- What is the role of near-infrared spectroscopy technology when inserting a peripheral VAD?
- What is the most appropriate method of teaching peripheral VAD insertion?
Group 4:
- What is the most appropriate maintenance strategy to reduce the risk of infection?
- What is the most appropriate maintenance strategy to reduce the risk of occlusion?
- What is the most appropriate maintenance strategy to reduce the risk of dislodgment?
- What is the most appropriate maintenance strategy to reduce the risk of phlebitis/thrombosis?
Group 5:
- When is the removal of a PVAD indicated?
- Are there any complications related to removal? What strategies can minimize such complications?
A summary of the results
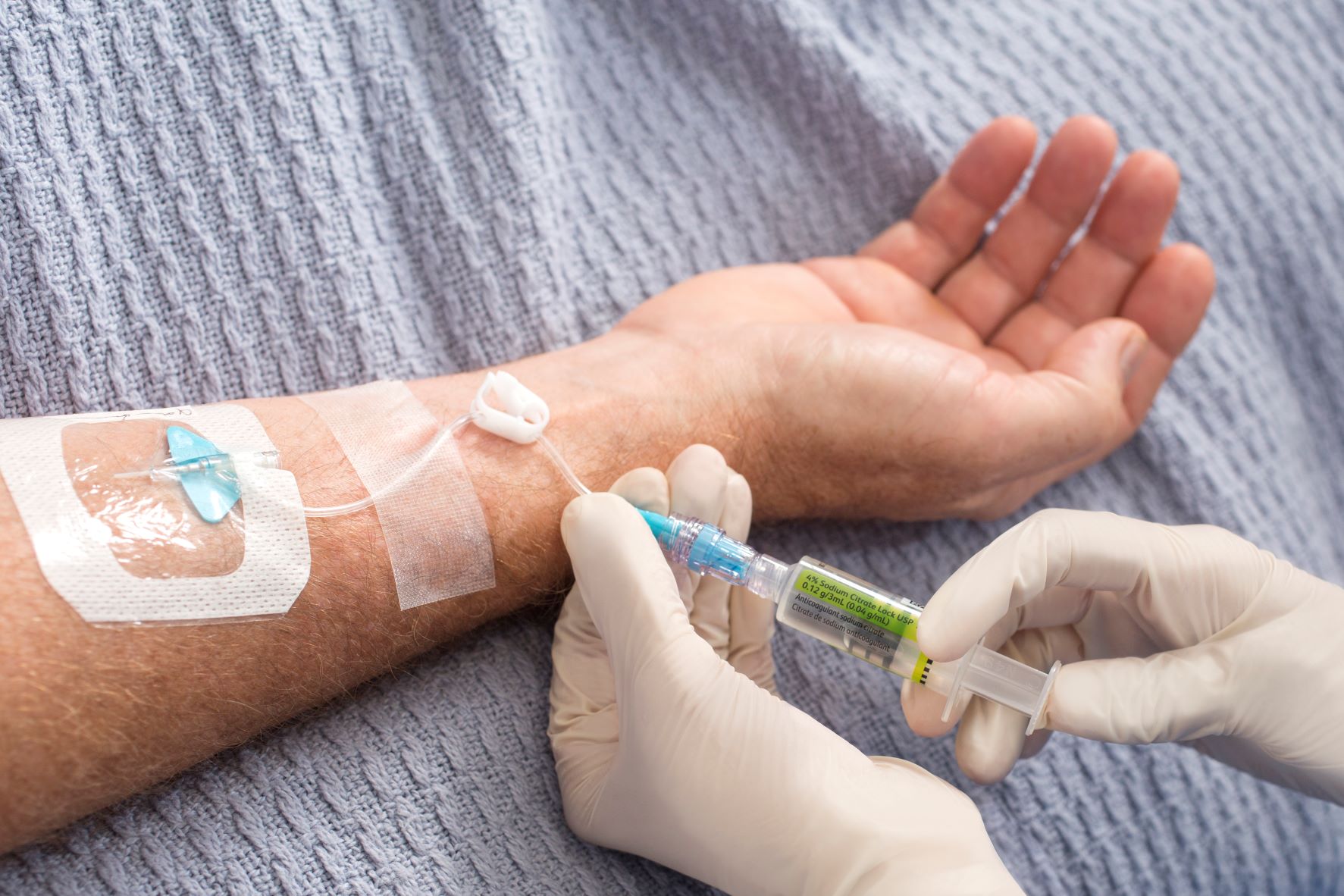
The panel defined peripheral VADs as catheters whose tip is located in the venous system but outside the superior vena cava, the right atrium and the inferior vena cava.1 They also classified them as short peripheral catheters (<6 cm), long peripheral catheters (6–15 cm) and midline catheters (>15 cm) based on their length. SPCs were further classified as ‘simple’ or ‘integrated’ based on their design and material.
The circumstances of PVAD usage were also indicated by the panel, as were the situations in which PVAD usage was contraindicated. Specific PVADs and their indications based on the expected duration of treatment were also defined.
Recommendations for PVAD insertion, skin preparation, dressing and securement for peripheral access longer 48 hours, were also made by the panel. Other recommendations included adopting ‘insertion bundles’ and skin preparation using of 2% chlorhexidine in 70% isopropyl alcohol, with 30 sec. of friction and drying for 30 sec.1
For maintenance, the panel recommended using 2% chlorhexidine alcohol to disinfect needle-free connectors and exit site disinfection for dressing change procedures, to help prevent infections. They recommended flushing PVADs with normal saline to reduce the risk of occlusion. The panel advised that dislodgement may be minimized by inserting PVADs in the forearm or upper arm, and the use of suture-less devices, semipermeable transparent dressing or cyanoacrylate glue. Avoiding micro-movements of the device is recommended to reduce the risk of phlebitis.1
Reasons for removal include: device no longer required or appropriate, device failure, a device inserted in emergency conditions or removal requested by the patient.1
Conclusion
The ERPIUP consensus document, developed by a panel of European experts, offers an overview of the current recommendations about the indication, insertion, management and removal of PVADs. While some of these may change over time with new scientific evidence generated in this area, the recommendations still offer appropriate strategies to optimise patient safety, clinical efficacy and cost-effectiveness.
Read about the study and the full recommendations here.
For more on our participation in the previous editions of WoCoVA