Chemotherapy: How do TIVAPs measure up to other CVADs?
Choice of CVAD in intravenous chemotherapy administration
Even though the advantages of central venous access devices (CVADs) over peripheral venous access devices (PVADs) for delivering chemotherapy have been demonstrated, there have been concerns about possible complications.
Thrombotic complications in CVADs may lead to life-threatening sequelae. Premature CVAD removal can deregulate chemotherapy cycles, possibly jeopardising clinical outcomes. Patients undergoing chemotherapy are especially vulnerable to healthcare-associated infections (HAIs) because they are immunocompromised. The mortality rate of central line-associated bloodstream infections (CLABSIs) ranges from 12–25% in the United States, making them one of the most deadly kinds of HAIs1.
Until recently, there was a lack of high-quality scientific evidence on the choice of CVAD used for administering intravenous (IV) chemotherapy. Different CVADs may offer varying levels of safety, quality of life (QoL) and cost-effectiveness.
Assessment of four CVADs
Yeow et al. carried out a systematic review and meta-analysis of randomised controlled trials (RCTs) assessing the following types of CVADs2:
- Non-tunnelled central venous catheter (CVC)
- Peripherally inserted central catheter (PICC)
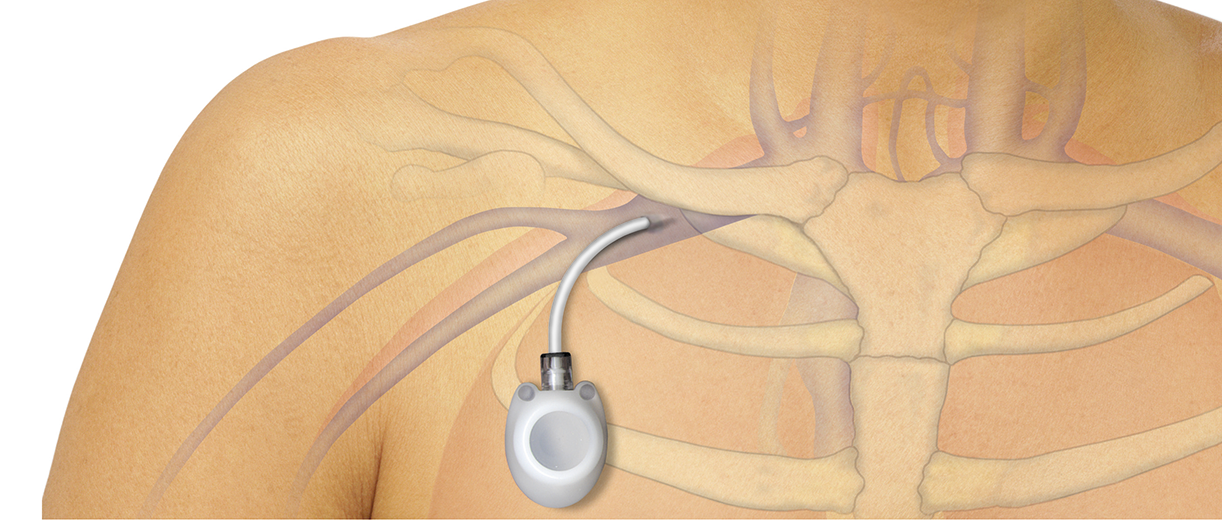
- Totally implantable venous access port (TIVAP)
- Tunnelled CVC
The above types of CVADs were compared based on: complication rates, QoL and cost-effectiveness. The authors included 11 RCTs (12 studies) with a total of 2,585 patients, of which a majority were women, aged 36–70 years.
Results
Catheter-related complications: How do TIVAPs compare?
In this general meta-analysis, TIVAPs were found to have lower rates of overall complications when compared with PICCs and tunnelled CVCs. TIVAPs were also associated with reduced rates of device removal due to complications when compared with the other three CVADs.
PICCs, tunnelled CVCs and non-tunnelled CVCs had higher rates of thrombotic complications than TIVAPs. Whereas, mechanical complications occurred at rates ranging from highest to lowest: non-tunnelled CVCs, PICCs, TIVAPs and then tunnelled CVCs.
Patients with PICCs inserted were found to have the lowest probability of developing infection (i.e., CLABSIs) compared with patients with the three other types of CVADs.
Quality of life: How do TIVAPs compare?
In terms of QoL, it was reported that TIVAPs led to less restriction in daily activity when compared with tunnelled CVCs. Using scores on the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30, there was no difference found in QoL between TIVAPs and PICCs.
Cost-effectiveness: How do TIVAPs compare?
It was estimated that TIVAPs had a lower total cost than tunnelled CVCs. However, PICCs were found to have a higher total cost when compared with TIVAPs, when accounting for total dwell time.
More on this topic: PICC line or port catheter: Which one for cancer patients?
What do these results mean for chemotherapy?
According to the results of this meta-analysis, TIVAPs were shown to have the highest safety profile of the four CVADs evaluated. In spite of the higher rates of CLABSIs with TIVAPs compared with PICCs, the authors recommend using TIVAPs as the standard of care for patients receiving IV chemotherapy. However, they acknowledge that PICCs may be used in certain situations because they have a shorter wait time than TIVAPs.
Learn more about this systematic review and meta-analysis from Yeow et al.: Read the full study
References
- Centers for Disease Control and Prevention (CDC). Vital signs: central line-associated blood stream infections–United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248.
- Yeow M, Soh S, Yap R, et al. A systematic review and network meta-analysis of randomized controlled trials on choice of central venous access device for delivery of chemotherapy. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1184-1191.e8. doi:10.1016/j.jvsv.2022.03.007
This list of references to third-party peer-reviewed material and the sites they are hosted on are provided for your reference and convenience only, and do not imply any review or endorsement of the material or any association with their operators. The Third-Party References (and the Web sites to which they link) may contain information that is inaccurate, incomplete, or outdated. Your access and use of the Third Party Sites (and any Web sites to which they link) is solely at your own risk.
BD-79050