Implementation of a standardized optimal procedure for peripheral venous catheters’ management: Results from a multidimensional assessment
This post is intended for a United Kingdom audience only .
Right device selection and end-user training are crucial factors when developing a procedure for peripheral vascular access management (PVAM)1.
Schettini F, Ferrario L, Foglia E, et al. The implementation of a standardized optimal procedure for peripheral venous catheters’ management: Results from a multi-dimensional assessment. PLoS One. 2022;17(1):e0263227. doi: 10.1371/journal.pone.0263227
Introduction
Peripheral venous catheters (PVCs) are frequently used in acute care settings1. However, 35–50% of PVCs don’t reach their intended dwell time and will need to be replaced prematurely2. Catheters fail due to complications such as: phlebitis, infiltration, occlusion/ mechanical failure, dislodgment or infection2.
Appropriate choice of device and effective training on peripheral vascular access management (PVAM) may contribute to positive clinical outcomes1.
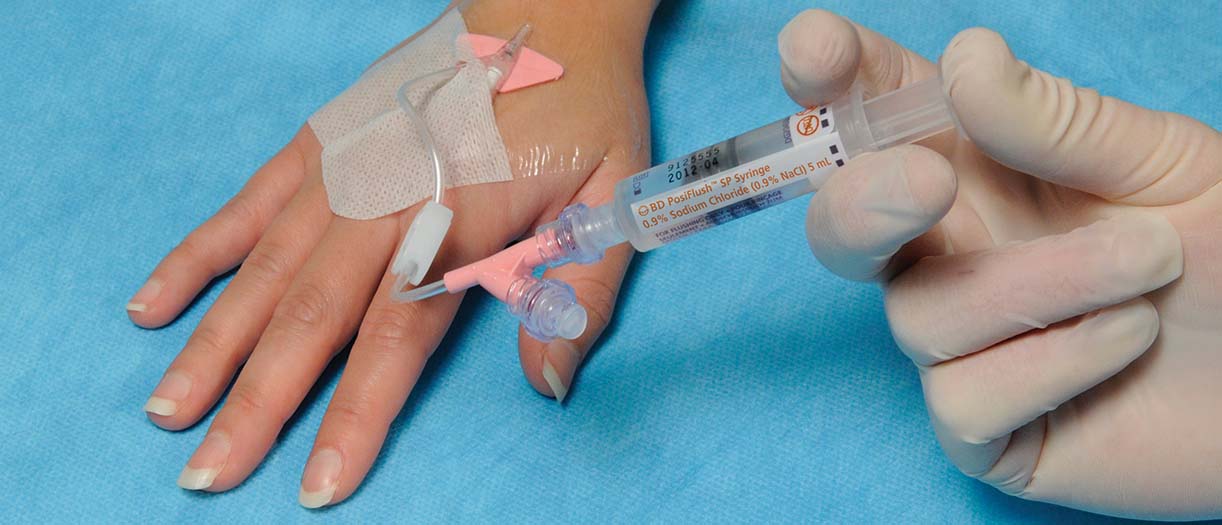
According to the literature3–6, using ChloraPrep™ Cutaneous Solution before catheter insertion rapidly kills microorganisms and its antimicrobial effect can last at least 48 hours1. The BD Nexiva™ Closed IV Catheter System helps prevent complications, such as phlebitis, infiltration/extravasation, dislodgement, CRBSIs, but can also help prevent needlestick injuries7. Regularly flushing IV catheters with the BD PosiFlush™ Pre-Filled Saline Syringe may help eliminate blood reflux, maintain cannula patency, while reducing CRBSIs1.
Methods
A total of 380 adult patients at two Italian hospitals were enrolled in this prospective observational study. Out of them, 68 were assigned to the standardized optimal procedure (Scenario 1), which used all three of the above BD solutions. And 312 patients were assigned to Scenario 2, where one or two of the above three solutions was used.
A health technology assessment (HTA) analysis was performed to assess the clinical, economic and organizational impact of using all three solutions together versus using one or two of them.
Results
With the standardized optimal procedure (Scenario 1), 86.8% of cannulas were removed at the end of treatment compared with 39.4% for cannulas in Scenario 2. Total cost per intervention was €19.60 for Scenario 1 and €21.71 for Scenario 2. In terms of time to perform all PVAM-related activities, the standardized optimal procedure (Scenario 1) took 4.39 minutes versus 5.72 for Scenario 2.
Conclusions
This study demonstrates that implementing a standardized optimal procedure may lower the incidence of complications, while reducing catheterization attempts. Longer dwell times with shorter execution times for PVC procedures have the potential to increase operational efficiency, while leading to cost savings for the healthcare facilities concerned.
ChloraPrep™ prescribing and technical information
References
- Schettini F, Ferrario L, Foglia E, et al. The implementation of a standardized optimal procedure for peripheral venous catheters’ management: Results from a multi-dimensional assessment. PLoS One. 2022;17(1):e0263227. doi:10.1371/journal.pone.0263227
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189-203. doi:10.1097/NAN.0000000000000100
- Florman S, Nichols RL. Current Approaches for the Prevention of Surgical Site Infections. American Journal of Infectious Diseases. 3(1):51-61.
- Crosby CT, Mares AK. Skin antisepsis: past, present, and future. J Vasc Access Devices. 2001;6(1):26-31.
- Maunoury F, Farinetto C, Ruckly S, et al. Cost-effectiveness analysis of chlorhexidine-alcohol versus povidone iodine-alcohol solution in the prevention of intravascular-catheter-related bloodstream infections in France. PLoS One. 2018;13(5):e0197747. doi:10.1371/journal.pone.0197747
- Guenezan J, Drugeon B, O’Neill R, et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, combined or not with use of a bundle of new devices, for prevention of short-term peripheral venous catheter-related infectious complications and catheter failure: an open-label, single-centre, randomised, four-parallel group, two-by-two factorial trial: CLEAN 3 protocol study. BMJ Open. 2019;9(4):e028549. doi:10.1136/bmjopen-2018-028549
- González López JL, Arribi Vilela A, Fernández del Palacio E, Olivares Corral J, Benedicto Martí C, Herrera Portal P. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126. doi:10.1016/j.jhin.2013.10.008
This list of references to third-party peer-reviewed material and the sites they are hosted on are provided for your reference and convenience only, and do not imply any review or endorsement of the material or any association with their operators. The Third-Party References (and the Web sites to which they link) may contain information that is inaccurate, incomplete, or outdated. Your access and use of the Third Party Sites (and any Web sites to which they link) is solely at your own risk.


BD-68425