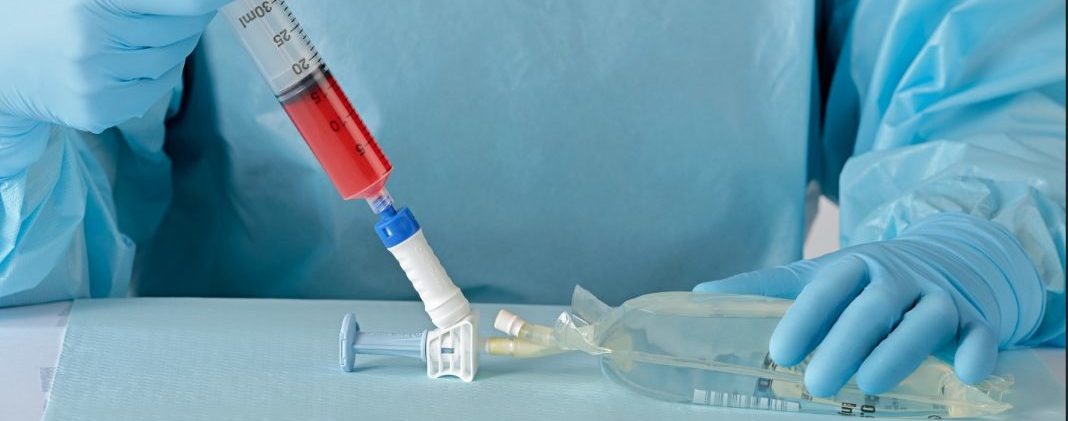
New clinic study: Reduce hazardous drug contamination with BD
Even in small concentrations, hazardous drug exposure can cause adverse effects such as internal organ damage, genetic mutations, increased rates of cancer and higher rates of reproductive issues.1-6
Whether you work in the pharmacy or the hospital, you may risk hazardous drug exposure on countertops, computers, door handles, biological safety cabinets and even the floor.7-9
Learn more about preventing hazardous drug contamination and exposure: Three ways to better protect healthcare workers from exposure to hazardous drugs
Testing the impact of closed-system transfer devices with the BD PhaSeal™ Optima
Closed-system transfer devices (CSTDs) are designed to prevent hazardous drug contamination through leakproof and airtight hazardous drug transfer.1
A recent study across two infusion centres at Emory Healthcare’s Winship Cancer Institute in Atlanta, Georga, USA assessed the changes in surface contamination with the introduction of the BD PhaSeal™ Optima Closed-System Transfer Device.1
Wipe samples from 29 locations at each infusion centre were taken prior to and a year following the introduction of the BD PhaSeal™ Optima.1
The authors found that hazardous drug contamination was reduced by 46% following the incorporation of the BD PhaSeal™ Optima.1
“Hazardous drugs are by nature harmful to anyone who comes into contact with them, but they are also a necessary part of health care to treat patients fighting cancer and other deadly diseases,”10 said Dr. Klaus Hoerauf, Vice President of Global Medical Affairs for Medication Delivery Solutions at BD. “Through our latest innovations in CSTD technology and rapid monitoring programs in hazardous drug solutions, we can help facilities protect their greatest assets – their teams of health care workers – while improving clinical workflow.”10
Learn more about how you can prevent hazardous drug contamination: Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination
How does the BD® HD Check compare to traditional detection methods?
The second goal of the Emory Healthcare study was to test the result validity of the BD® HD Check, a point-of-care lateral flow immunoassay hazardous drug testing device.1 Traditional liquid chromatography with mass spectrometry (LCMS/MS) analyses were compared against the results of the BD® HD Check.1
The study reported that the BD HD® Check readings were “overwhelmingly consistent with quantitative analyses.”1 The correlation of the accuracy of the BD® HD Check against traditional LCMS/MS analyses was 90.7%.1 However, if only data falling within its limits of detection (LOD) are considered, the accuracy rose to 97.7%.1
Currently, the BD® HD Check is the only rapid hazardous drug detection system that can provide results in less than 10 minutes, detecting three common substances: methotrexate, cyclophosphamide and doxorubicin.1
“Quantitative wipe sampling presents a significant time and cost barrier, resulting in routine monitoring adherence rates around 25%,”10 said Erich Brechtelsbauer, PharmD, MS, Assistant Director of Pharmacy at Emory Healthcare and the Winship Cancer Institute and author of the study. “Having a rapid test that can identify the presence of hazardous drug residue in real-time may help health care workers quickly implement corrective action planning to reduce contamination and potential exposure while also minimizing costly quantitative testing and improving routine monitoring.”10
Learn more about the BD® HD Check: Analysis of chemical contamination by hazardous drugs with BD® HD Check System in a tertiary hospital
#Hazardous drug contamination
References
- Brechtelsbauer E. Identification and reduction of hazardous drug surface contamination through the use of a novel closed-system transfer device coupled with a point-of-care hazardous drug detection system. Am J Health Syst Pharm. 2022 Nov 12;zxac336. Doi: 10.1093/ajhp/zxac336.
- Sotaniemi EA, Sutinen S, Arranto AJ, et al. Liver damage in nurses handling cytostatic agents. Acta Med Scand. 1983;214(3):181-189. doi:10.1111/j.0954-6820.1983.tb08593.x
- McDiarmid MA, Oliver MS, Roth TS, Rogers B, Escalante C. Chromosome 5 and 7 abnormalities in oncology personnel handling anticancer drugs. J Occup Environ Med. 2010;52(10):1028-1034. doi:10.1097/JOM.0b013e3181f73ae6
- Cavallo D, Ursini CL, Perniconi B, et al. Evaluation of genotoxic effects induced by exposure to antineoplastic drugs in lymphocytes and exfoliated buccal cells of oncology nurses and pharmacy employees. Mutat Res. 2005;587(1-2):45-51. doi:10.1016/j.mrgentox.2005.07.008
- Skov T, Maarup B, Olsen J, Rørth M, Winthereik H, Lynge E. Leukaemia and reproductive outcome among nurses handling antineoplastic drugs. Br J Ind Med. 1992;49(12):855-861. doi:10.1136/oem.49.12.855
- Lawson CC, Rocheleau CM, Whelan EA, et al. Occupational exposures among nurses and risk of spontaneous abortion. Am J Obstet Gynecol. 2012;206(4):327.e1-e8. doi:10.1016/j.ajog.2011.12.030
- Connor TH, Zock MD, Snow AH. Surface wipe sampling for antineoplastic (chemotherapy) and other hazardous drug residue in healthcare settings: methodology and recommendations. J Occup Environ Hyg. 2016;13(9):658-667. doi:10.1080/15459624.2016.1165912
- Janes A, Tanguay C, Caron NJ, Bussières JF. Environmental Contamination with Cyclophosphamide, Ifosfamide, and Methotrexate: A Study of 51 Centres. Can J Hosp Pharm. 2015 Jul-Aug; 68(4): 279-289. Doi: 10.4212/cjhp.v68i4.1467.
- Walton A, Bush MA, Douglas C, Allen DH, Polovich M, Spasojevic I. Surface contamination with antineoplastic drugs on two inpatient oncology units. Oncol Nurs Forum. 2020;47(3):263-272. doi:10.1188/20.ONF.263-272
- Becton Dickinson. Press Release: New Clinical Use Study Finds BD Technology Can Help Reduce Hazardous Drug Contamination, Speed Up Sampling Times in Health Care Settings. Published 23 February 2023. Accessed 3 April 2023 at: https://news.bd.com/2023-02-23-New-clinical-use-study-finds-BD-technology-can-help-reduce-hazardous-drug-contamination,-speed-up-sampling-times-in-health-care-settings.
This list of references to third-party peer-reviewed material and the sites they are hosted on are provided for your reference and convenience only, and do not imply any review or endorsement of the material or any association with their operators. The Third-Party References (and the Web sites to which they link) may contain information that is inaccurate, incomplete, or outdated. Your access and use of the Third Party Sites (and any Web sites to which they link) is solely at your own risk.
![]()