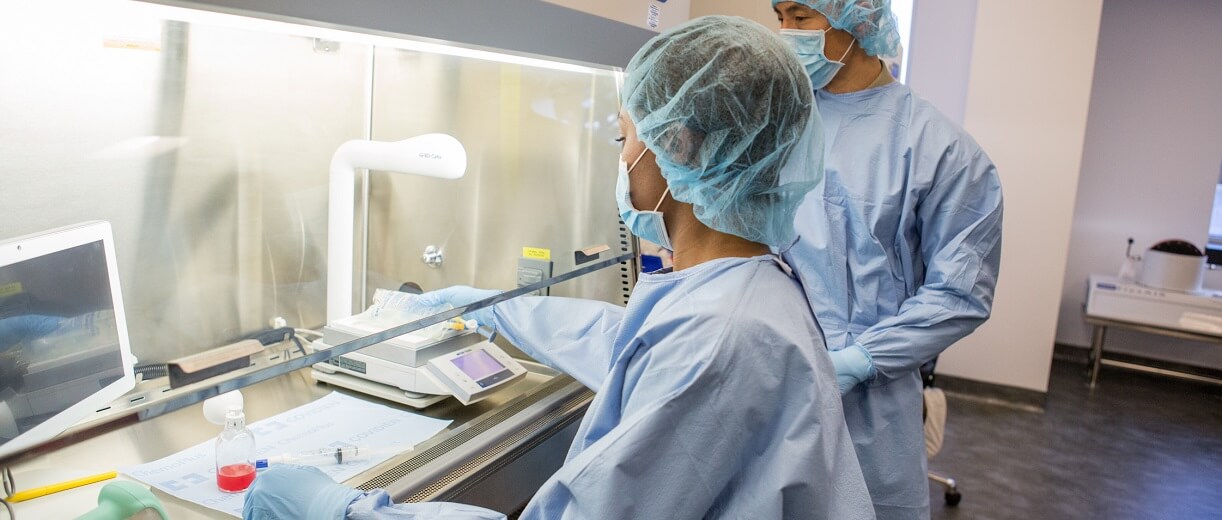
The European Union reaches a political deal to protect healthcare workers from reprotoxic substances
The members of the European Parliament have secured a deal allowing for the inclusion of reprotoxic substances in the fourth revision of the Carcinogens and Mutagens Work directive (CMD4). As a consequence, the directive will now be renamed Carcinogens, Mutagens and Reprotoxic substances Directive (CMRD).1 A binding occupational limit value for 11 substances will be included in the annex to the Directive.1
The inclusion of reprotoxic substances in the Directive had been demanded by groups like the Stop Cancer at Work campaign.2
Reprotoxic substances and healthcare workers
A reprotoxic substance or preparation, when inhaled, ingested or taken through the skin, may produce or increase the incidence of non-inheritable adverse effects in the progeny and/or the impairment of male or female reproductive functions or capacity.3 The exposure of healthcare workers like oncology/head nurses and hospital pharmacists, to reprotoxic drugs with anti-tumoral activity is an area of great concern that the new directive seeks to address. The Commission shall, after consulting the stakeholders, prepare Union guidelines and standards of practice for the preparation, administration and disposal of hazardous medicinal products at the workplace.1
What can healthcare workers expect?
The agreement aims to provide healthcare workers with greater preventive measures against exposure to reprotoxic drugs at the workplace. Healthcare workers can also expect to receive sufficient and appropriate training for increased protection.1
References
1 European Parliament. Political deal on protecting workers from hazardous substances. https://www.europarl.europa.eu/news/pt/press-room/20211208IPR19046/political-deal-on-protecting-workers-from-hazardous-substances. Published Dec 8, 2021. Accessed Dec 16, 2021.
2 Stop Cancer at Work Campaign. Reprotoxins and Firefighters. https://www.stopcanceratwork.eu/. Published Sept 22, 2020. Accessed Jan 13, 2022.
3 French Agency for Food, Environmental and Occupational Health and Safety (ANSES). Carcinogenic, mutagenic and reprotoxic substances (CMRs) – Definition and regulatory framework. https://www.anses.fr/en/content/carcinogenic-mutagenic-and-reprotoxic-substances-cmrs. Last updated Sept 22, 2016. Accessed Jan 13, 2022.
This list of references to third-party peer-reviewed material and the sites they are hosted on are provided for your reference and convenience only, and do not imply any review or endorsement of the material or any association with their operators. The Third-Party References (and the websites to which they link) may contain information that is inaccurate, incomplete, or outdated. Your access and use of the Third Party Sites (and any websites to which they link) is solely at your own risk.
BD-53160