Accurate liquid-based cytology testing that enhances patient care

Discover BD SurePath™ Liquid-based Pap Test
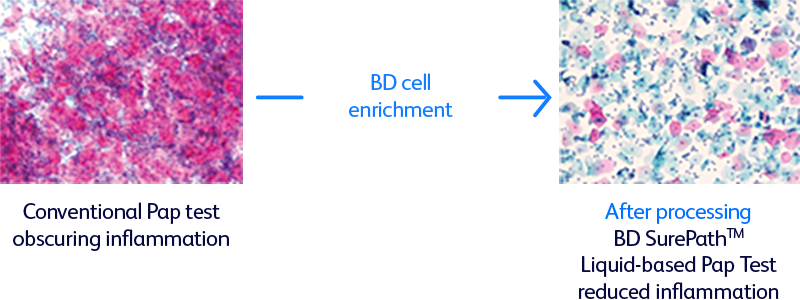
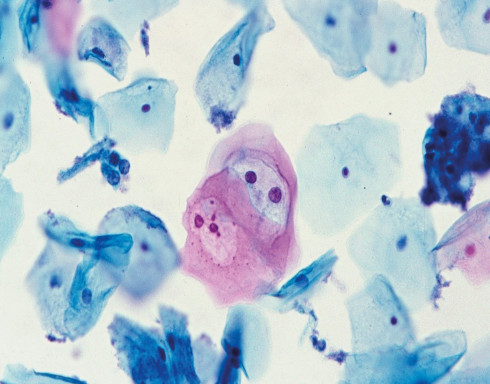
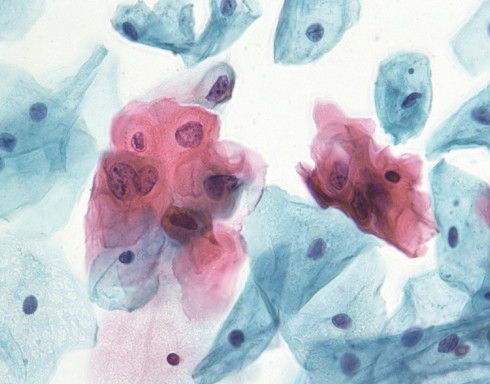
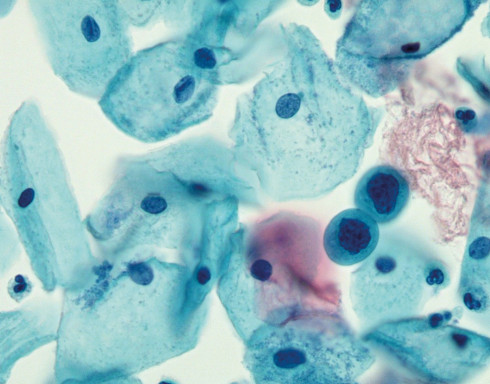
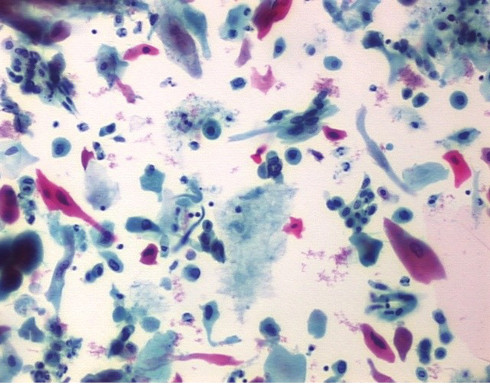
The BD SurePath™ Liquid-based Pap Test is an FDA and CE-marked cytology method with higher cervical disease detection and a lower unsatisfactory rate compared to conventional cytology and other liquid-based cytology (LBC) devices.1
![]()
Simple and efficient cell collection that ensures sample quality
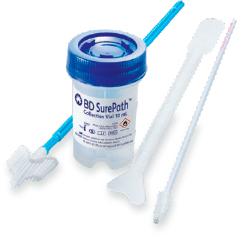
- The head of the collection device is deposited directly into the BD SurePath™ Collection Vial so 100% of the collected cells are sent to the laboratory for analysis – unlike the rinsing method which can result in up to 37% of cells being discarded.2,3
- The cells are preserved in an ethanol solution for optimal slide preparation in the lab.4
- The same sample can be used for cytology testing, immunocytochemistry, and HPV testing.4-6
See how BD SurePath™ can help you to collect cervical samples of high quality for cytology and molecular testing.