BD Onclarity™ HPV Assay offers a reliable DNA-based PCR assay

The BD Onclarity™ HPV Assay reduces the number of false-positive and false-negative results
The BD Onclarity™ HPV Assay is designed to reduce the number of false-positive and false-negative results by using gene-specific PCR primers and an internal control so you can be confident in the results that you get.1
Fewer false-positives could mean fewer unnecessary colposcopies.
Fewer false-negatives could mean fewer missed cervical disease cases.
The BD Onclarity™ HPV Assay provides:
Excellent detection of HPV infections with multiple genotypes thanks to genotype-specific primers and probes (instead of consensus primers).1,2
Minimal risk of false-positive results due to no cross-reactivity with low-risk HPV genotypes.1,3,4
Minimal risk of false-negative results by:
- Including an internal control to provide confidence that sample collection was adequate and that the entire assay process functioned properly.1
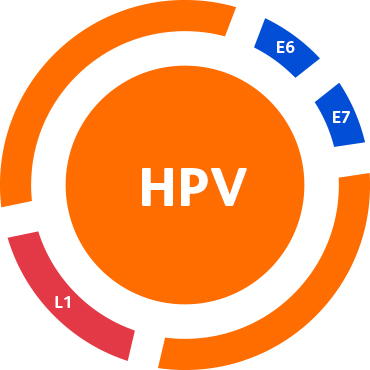
- Targeting the E6/E7 region of the HPV viral genome rather than the L1 region, which can undergo deletion during HPV DNA integration.5,6
Similar sensitivity and specificity on self-collected and clinician-collected samples, as opposed to signal amplification-based and mRNA-based HPV assays that seem to have a lower sensitivity on self-collected samples.7-10
Expand on our reliable cervical cancer screening solutions with BD SurePath™ Liquid-based Pap Test for accurate and definitive cytology results that enhance patient care.
BD SurePath™ Liquid-based Pap
Test
BD Onclarity™ HPV Assay offers a reliable DNA-based PCR assay design providing high sensitivity and specificity for results that you can trust.1
DNA, deoxyribonucleic acid; HPV, human papillomavirus; Pap, Papanicolaou; PCR, polymerase chain reaction.
1. BD Onclarity™ HPV Assay EU Package Insert [8089899].
2. Wright TC et al. Am J Clin Pathol. 2014;142(1):43–50.
3. Preisler S et al. BMC Cancer. 2016;16:510.
4. Ejegod DM et al. Papillomavirus Res. 2016;2:31–7.
5. Vaughan LM and Malinowski DP. Rev Bras Ginecol Obstet. 2019;41(5):357–9.
6. Arroyo Mühr LS et al. J Gen Virol. 2020;101:265–70.
7. Rohner E et al. J Clin Microbiol. 2020;58(3):e01443-19.
8. Polman NJ et al. Lancet Oncol. 2019;20(2):229–38.
9. Arbyn M et al. BMJ. 2018;363:k4823.
10. Arbyn M et al. Lancet Oncol. 2022;23(7): 950–60.