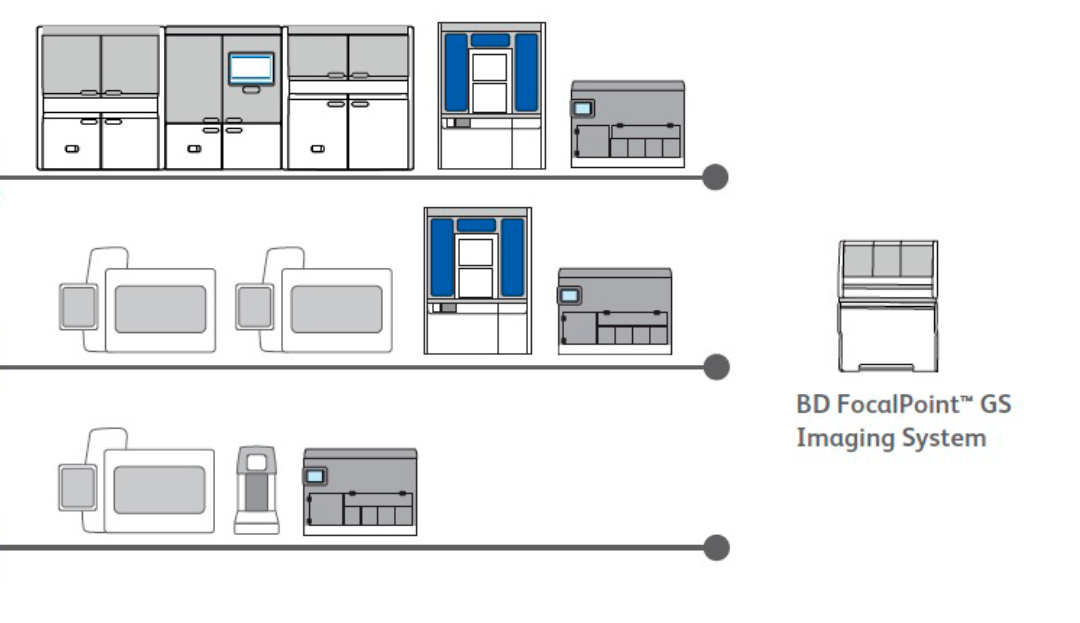
Fully integrated end-to-end cervical cancer screening solutions

Cervical cancer testing designed for the future.
BD offers a full suite of advanced cervical cancer screening products and services that empower clinicians and laboratories to meet the needs of the rapidly changing cervical cancer screening landscape and adapt to evolving guidelines.
A complete portfolio of collection devices, assays and instruments to keep you ahead of the curve.
Flexible solutions so you can adapt to all screening paradigms.
Versatile, integrated and scalable instrumentation that fits your laboratory’s size.
Fully integrated end-to-end solutions.
ASC-US, atypical squamous cells of undetermined significance; DNA, deoxyribonucleic acid; FDA, Food and Drug Administration; HPV, human papillomavirus; LBC, liquid-based cytology; Pap, Papanicolaou; PCR, polymerase chain reaction.
1. BD Onclarity™ HPV Assay Package Insert (8089899).
2. BD SurePath™ Collection Vial Package Insert (500017013).
3. Nance KV. Diagn Cytopathol. 2007;35(3):148–53.