HPV Self-collection with BD Onclarity™ HPV Assay

BD Onclarity™ HPV Assay: validated for use on self-collected samples both at home and in the clinic
Self-collection is gaining adoption and may become a method of choice for the future of cervical cancer screening programmes, not only to increase access to screening, but also as a convenient and cost-effective method for routine HPV screening.1-6
The BD Onclarity™ HPV Assay provides a fully-validated, automated and performant solution to answer the growing need for self-collection in HPV testing.7,8
The simplest way to process self-collected HPV samples NEW
The BD COR™ System redefines HPV self-collection processing with a dry sample workflow that eliminates complexity and manual burden. With only one manual step – loading the sample tube – labs gain true automation, from primary sample to result.10
An enhanced workflow for HPV self-collected samples
Dry transport simplifies logistics, while onboard rehydration enables seamless, automated lab processing – a scalable, hands-free, and accurate solution without manual intervention.
Vaginal sample is self-collected at doctor’s office or at home with the safe and proven FLOQSwabs® for HPV self collection.10
Samples are transported dry with 30-day stability under extreme temperatures*, allowing ample time for transit and processing while minimising the risk of rejection.9
*Including up to 4 freeze/thaw cycles
or up to 5 days at 45°C9
Simply load the BD Onclarity™ HPV Self Collection Tube into the rack, load into the BD COR™ System, and walk away. The system automatically dispenses diluent and performs all preanalytical steps, reducing the risk of human error.10
One automated workflow for all sample types
The BD COR™ System uses a single automated workflow for both self-collected and clinician-collected samples.10

BD COR™ PX
Performs all preanalytical steps without human intervention.
This means no manual:
- Rehydration
- Vortexing
- Swab transfer
- Pipetting
- Sample sorting
- Uncapping/recapping
- Aliquoting
- Warming/cooling
BD COR™ GX
- Automatically transfers samples from the PX to GX module
- High-throughput analysis for extended HPV genotyping
- Automates reporting
BD Onclarity™ HPV Assay with self collection supports patients and labs
Empowering greater workflow efficiency for labs and expanding access to life-saving screening for patients.
Self collection option at home or in a doctor’s office
BD Onclarity™ HPV Assay with self-collection offers convenience, privacy, and has comparable performance to clinician-collected samples,8,11 expanding access to cervical cancer screening without compromising diagnostic confidence.
Different sample types, one unified workflow
All samples, self-collected or not, follow a streamlined, barcode-driven workflow.10 This simplifies operations, lowers training demands, and ensures consistent, reliable results regardless of collection method.
Clinically validated for broad sample stability
Self-collected samples are transported dry and remain stable for 30 days across 2°C to 30°C, offering flexibility in transport and reducing the chance of rejection. They can also tolerate 5-day excursions at 45°C, ensuring reliability even in harsh shipping conditions. Optimise logistics without compromising quality.9
Integrated pre-analytics, no sample transfer
All pre-analytical and analytical steps are fully integrated and automated—including onboard rehydration.10 With a workflow requiring just one manual step, labs can reduce training, eliminate manual handling, and ensure consistent results—even with limited staffing.
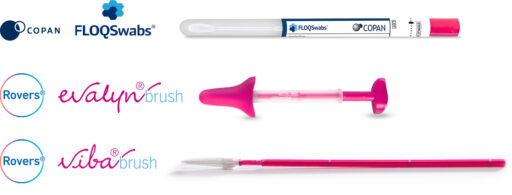
Multiple collection options to support cervical cancer screening
The BD Onclarity™ HPV assay is compatible with various collection devices.
Self collected samples (at home or in clinic):
BD Copan FLOQSwabs® for HPV Self Collection
Clinician-collected samples:
BD SurePath™ and PreservCyt® vials
Transport and storage conditions for self-collected vaginal specimens in BD Onclarity™ HPV Self Collection Tube
aIncludes time in the home, in transit, in the lab, and/or on the deck of the BD COR™ System prior to addition of diluent
bIncluding up to 4 freeze/thaw cycles or up to 5 days at 45oC
Ordering information
Equivalent sensitivity on self-collected and clinician-collected samples
Lowest proportion of invalid results
Self-collection with the BD Viper™ LT System
The importance of self-collection
* Ratio of sensitivity for self-collected samples to sensitivity for clinician-collected samples.
† With up to 6 days exposure at 40 ºC.
ASC-US, atypical squamous cells of undetermined significance; CIN2+, cervical intraepithelial neoplasia grade 2 or higher; FDA, Food and Drug Administration; HPV, human papillomavirus; PCR, polymerase chain reaction.
1. Lozar T et al. Int J Womens Health. 2021;13:841–59.
2. Arbyn M et al. Clin Microbiol Infect. 2021;27(8):1083–95.
3. Arbyn M et al. BMJ. 2018;363:k4823.
4. Yeh PT et al. BMJ Glob Health. 2019;4(3):e001351.
5. Hawkes D et al. Cancers. 2020;12(4):1053.
6. World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention – Second Edition. 2021.
7. Rohner E et al. J Clin Microbiol. 2020;58(3):e01443-19.
8. Martinelli M, Latsuzbaia A, Blond J. Et al. Performance of BD Onclarity HPV assay on FLOQSwabs vaginal self-samples.Microbial Spectrum. 2024. doi: 10.1128/spectrum.02872-23.
9. BD Onclarity™ HPV Self Collection Kit [package insert]. Sparks, MD: Becton, Dickinson and Company; 2025.
10. BD Onclarity™ HPV Assay for the BD COR™ System [package insert]. Sparks, MD: Becton, Dickinson and Company; 2025.
11. BD Onclarity™ HPV Assay US Package Insert [8089894]. Becton, Dickinson and Company; 2025.
12. Saville M et al. J Clin Virol. 2020;127:104375.